More deaths, no benefit from Malaria drug touted by Trump
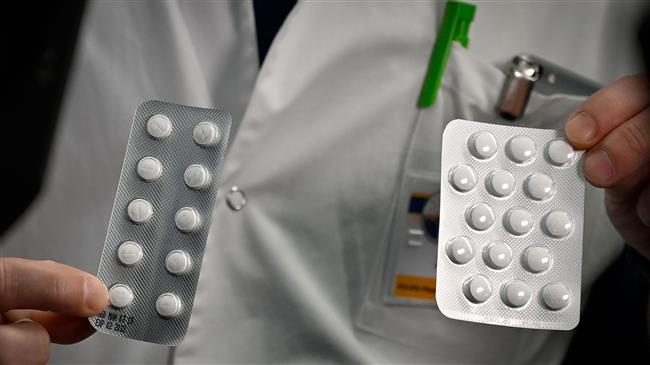
An anti-Malria drug touted by US President Donald Trump has shown no benefit and even caused more deaths among COVID-19 patients compared to those not on the drug, according to a new study.
The nationwide study, financed by the National Institutes of Health and the University of Virginia, has inspected 368 male veterans with confirmed infection at Veterans Health Administration medical centers who died or were discharged by April 11.
About 28% who were given hydroxychloroquine plus usual care died, versus 11% of those getting routine care alone. About 22% of those getting the drug plus azithromycin died too, but the difference between that group and usual care was not considered large enough to rule out other factors that could have affected survival.
Hydroxychloroquine made no difference in the need for a breathing machine, either.
Researchers did not track side effects, but noted a hint that hydroxychloroquine might have damaged other organs. The drug has long been known to have potentially serious side effects, including altering the heartbeat in a way that could lead to sudden death.
Earlier this month, scientists in Brazil stopped part of a study testing chloroquine, an older drug similar to hydroxychloroquine, after heart rhythm problems developed in one-quarter of people given the higher of two doses being tested.
On Tuesday, NIH issued new treatment guidelines from a panel of experts, saying there was not enough evidence to recommend for or against chloroquine or hydroxychloroquine for COVID-19. But it also advised against using hydroxychloroquine with azithromycin because of the potential side effects.
Many doctors have been leery of the drug.
At the University of Wisconsin, Madison, “I think we’re all rather underwhelmed” at what’s been seen among the few patients there who’ve tried it, said Dr. Nasia Safdar, medical director of infection control and prevention.
Patients asked about it soon after Trump started promoting its use, “but now I think that people have realized we don’t know if it works or not” and needs more study, said Safdar, who had no role in the VA analysis.
The NIH and others have more rigorous tests underway.
(Source: AP)
Iran puts ‘Jam‑e Jam 1’ into orbit in milestone for national broadcasting
‘Colonial eradication of Palestine’: Iran condemns Israel’s West Bank annexation push
Thousands block Melbourne as Israeli president ends contentious Australia visit
Nearly 800 Lufthansa flights cancelled as pilots, cabin crew strike
Pezeshkian: US, Israel exploit Iran’s challenges without genuine concern
VIDEO | Press TV's news headlines
VIDEO | Chaos by design
Leader hails Iranians for disappointing enemies with multimillion rallies









 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website