Iran, Cuba’s COVID-19 vaccine project proceeds to clinical trial phase II
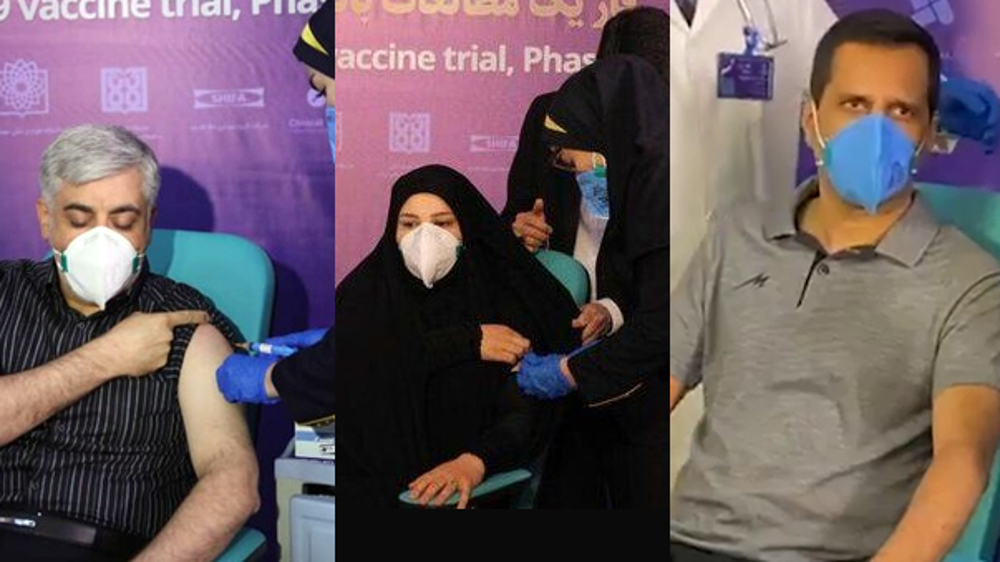
A coronavirus vaccine project being jointly pursued by Iran and Cuba has entered phase II of its clinical trials, with no side effects having been reported so far.
Cuba and Iran signed an agreement earlier this month to cooperate in the coronavirus vaccine project with the use of a technology that will be transferred to Iran by Havana.
The vaccine, known as Subrana 2, is now undergoing its human phase trials by the Cuban Finlay Institute and the Pasteur Institute of Iran.
The vaccine, which is the most advanced one among Cuba’s four other coronavirus vaccines, has so far shown no side effects, as the initial stages of its testing have been completed successfully.
Iran also launched human trials of its first domestic coronavirus vaccine late last month.
The vaccine, known as COViran Barekat, is being produced under the health protocols and guidelines announced by the Ministry of Health and Medical Education.
Mohammad Mokhber, the director of the Headquarters for Executing the Order of Imam Khomeini, which is developing the vaccine, said on Monday that the third stage of the trials would see the injection of the vaccine to 56 volunteers.
The homegrown vaccine, Mokhber said, has been tested on volunteers in previous stages successfully without causing any side effects.
“We are trying to increase the production volume of the vaccine to up to three million doses per month in the next month,” he said.
Iran will reach the production of 14 million doses of the vaccine within the next four months with the continuation of this trend, according to Mokhber.
This comes as Iran is under illegal sanctions imposed by the United States, which have hampered its access to medical equipment and pharmaceuticals and have complicated the process of importing vaccines from other countries.
A second domestic vaccine has also been approved by both the Iranian Food and Drug Administration and the Iranian National Committee for Ethics in Biomedical Research.
If it passes the first phase successfully, the acceptable dose of the vaccine will be determined, and based on the results of phase I, the second phase will begin with 500 people, according to the proposed protocol by the Razi Vaccine and Serum Research Institute.
The National Committee for Ethics in Biomedical Research and the senior experts will supervise the project in all stages of the trial.
Some 12 companies have so far applied to produce the vaccine, according to Kianoush Jahanpour, the head of the Information Center of the Ministry of Health.
Earlier this month, the Iranian Red Crescent Society (IRCS) canceled the import of 150,000 doses of the Pfizer vaccine after Leader of the Islamic Revolution Ayatollah Seyyed Ali Khamenei banned the purchases of vaccines produced by the United States and Britain.
President Hassan Rouhani said on Saturday that his government was doing its best to roll out mass vaccination in two months, with the priority given to medical staff and high-risk individuals.
Health officials on Wednesday confirmed 6,182 new cases of COVID-19 infection in the past 24 hours in Iran, bringing the total caseload to 1,348,316.
Health Ministry spokeswoman Sima-Sadat Lari said that 1,137,812 patients had so far recovered, but 4,214 remained in critical condition.
She also provided a daily death toll of 84.
US imposes ‘terrorist-grade sanctions’ on UN expert, ICC judges amid Gaza accountability drive
VIDEO | Press TV's news headlines
Senior Russian general shot and wounded in Moscow: Officials
UK ordered in 'milestone' court ruling to pay $570 million for colonial-era massacre
VIDEO | Defying the rubble, Gaza opens its first face-to-face school since start of war
‘Ready for next round’: Million-man rally in Yemen backs Gaza, resistance
FM Araghchi departs Muscat for Doha following nuclear talks with US
Israeli keeps killing more Palestinian civilians in Gaza amid relentless ceasefire violations














 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website