Iran develops ‘surgical assistant’ device, cutting breast cancer detection time to 15 seconds
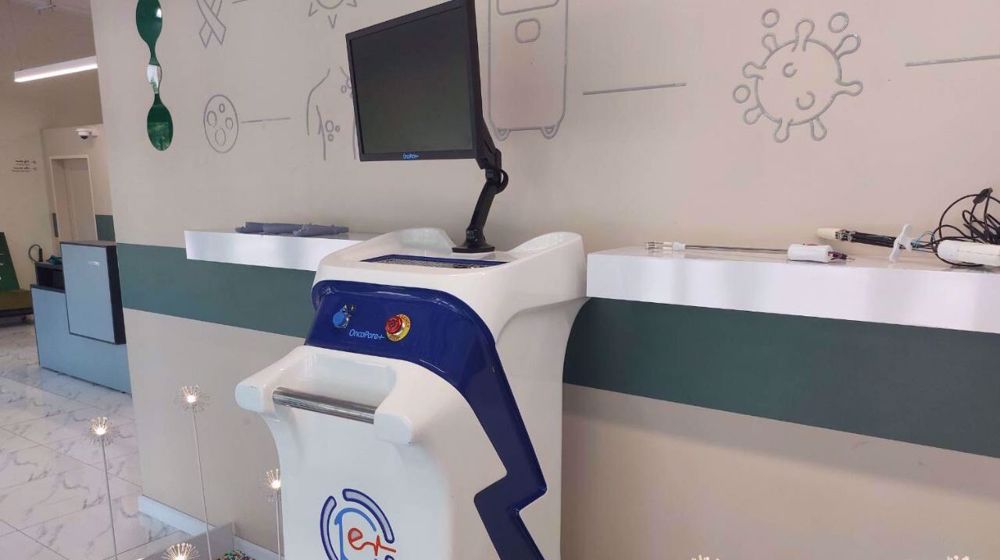
An Iranian knowledge-based company has developed a novel surgical assistant device designed to help surgeons identify cancerous margins during breast cancer operations, reducing diagnosis time from about 45 minutes to as little as 15 seconds.
In an interview with Mehr news agency on Saturday, Mohammad Hossein Yazdi, sales manager at Aria Health Nanosensors Company, said the Cancer Diagnostic Probe (CDP) device was the first of its kind in the world and is capable of identifying cancer margins during breast cancer surgery with high accuracy.
The CDP system functions as an intraoperative assistant, alerting surgeons to tissue margins suspected of containing cancer cells, even when they are just a few millimeters in size.
Utilizing a pathology-calibrated needle sensor, the device delivers results categorized as negative, suspicious, or positive, adhering to the latest standards set by the World Health Organization. The results appear on a monitor in just 15 seconds.
Yazdi further highlighted that clinical trials indicate the CDP can detect around 30% of cancer-infected margins that traditional frozen-section and permanent pathology methods might overlook.
In breast cancer surgeries, the CDP achieved an impressive accuracy rate of over 90%, with some trials showing results surpassing 93% when identifying precancerous or cancerous margins that standard methods failed to detect.
The company asserts that this capability could lower the number of contaminated margins left in patients by about 30%, even when standard pathology techniques have been applied.
Typically, frozen pathology—a common method used during surgery—can take up to 45 minutes and has an accuracy rate of around 70%.
In contrast, Yazdi noted that the CDP device significantly shortens decision-making for surgeons while enhancing diagnostic precision, ultimately reducing the likelihood of remaining cancer cells and the chance of local recurrence.
Citing global statistics, Yazdi stated that around one in five women worldwide experience a recurrence after breast cancer surgery, often in a more aggressive form.
In Iran, recurrence rates are estimated at about 30%, with a significant share attributed to limitations and errors in frozen pathology.
According to Yazdi, the use of the CDP device could potentially reduce at least 20% of these errors.
The device has been evaluated and endorsed by several of Iran’s leading breast surgeons, who have confirmed its effectiveness and are providing training on its use to surgical fellows.
The probe head of the device is disposable, a feature designed to prevent cross-contamination and enhance safety for patients and medical staff.
Yazdi further said the CDP currently has no domestic competitors and that no foreign product offers the same level of performance and application.
More than five patents related to the technology have been registered in the United States, and research findings from its clinical use continue to be published in international scientific journals.
The CDP device is registered in Iran’s “Iransakht” system at a price of 2.5 billion tomans, which includes a 40% subsidy for government medical centers. Disposable probe heads cost about 450,000 tomans each, with between one and five probes typically used per operation, depending on surgical conditions.
At present, around 10 medical centers across Iran are equipped with the device, including Shohadaye Tajrish Hospital and Imam Khomeini Hospital in Tehran, Maryam Hospital in Alborz Province, Seyed al-Shohada Hospital in Isfahan, and Shiraz Central Hospital.
Yazdi further noted that the company has not started exporting the device yet, primarily due to the hurdles associated with obtaining international regulatory approvals.
He explained that since the technology is completely new, overseas clinical trials are necessary, which entails high costs and the need to set up infrastructure in other countries.
VIDEO | Press TV's news headlines
VIDEO | 1979 Revolution: The long road to “no” to submission
Iran's nuclear chief urges Grossi to stick to his IAEA mandate after NPT jab
Iran will respond 'like never before', UN mission warns after Trump’s threats
Venezuela’s Rodríguez rejects US push to cut ties with China, Russia, Iran: US Intel.
Iraq's former PM Maliki rejects ‘blatant US interference’ after Trump warning
Iran, Saudi Arabia warn of 'dangerous consequences' of regional escalation
VIDEO | India, EU seal trade pact slashing tariffs to curb US reliance













 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website